
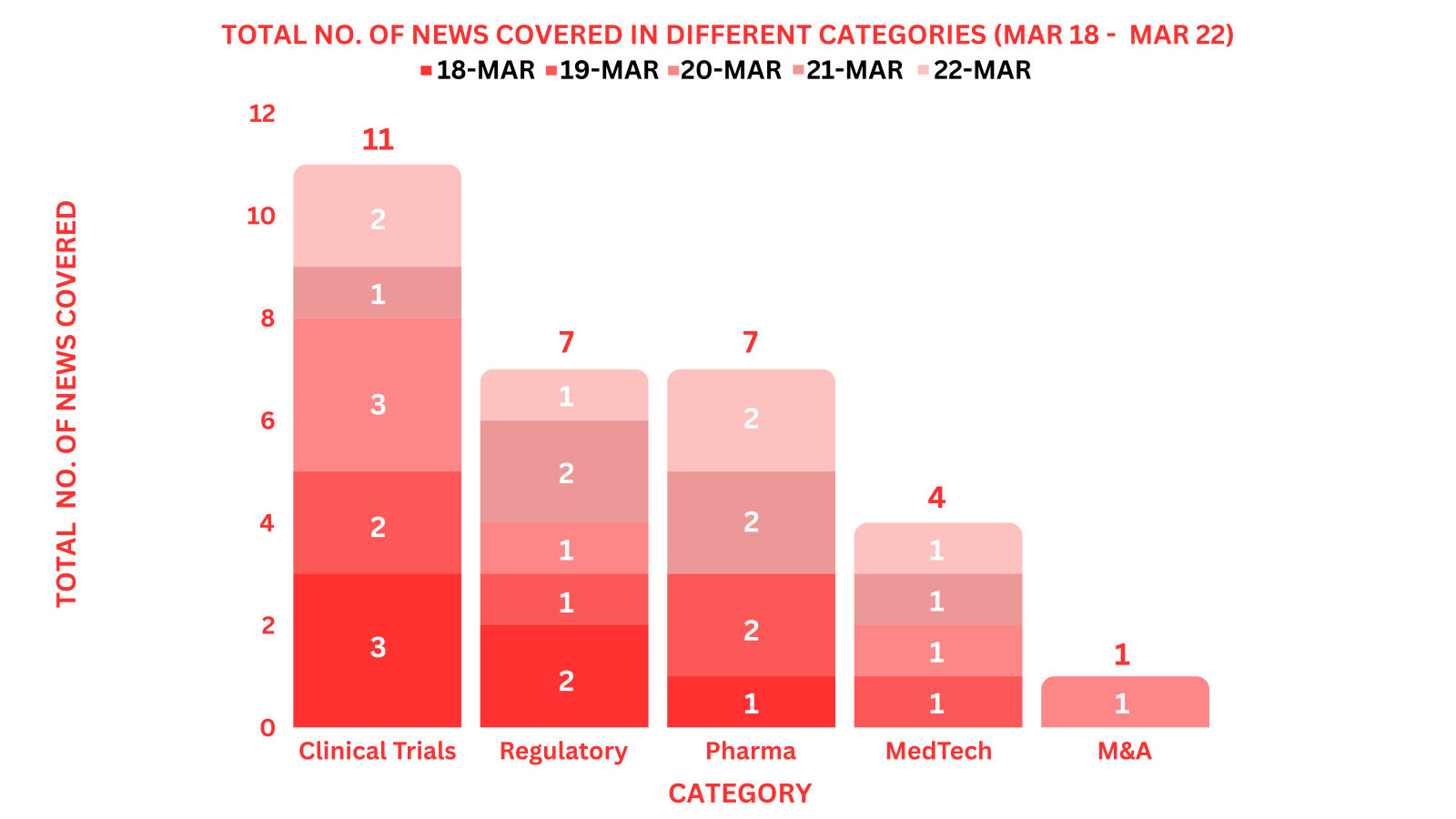
PharmaShots Weekly Snapshots (March 18 – March 22, 2024)
This week PharmaShots’ news was all about the updates on M&A, Pharma, Clinical Trials, Regulatory & MedTech. Check out our full report below:

Cybin Reports Initiation of the P-II Clinical Evaluation of CYB004 for the Treatment of Generalized Anxiety Disorder
Read More: Cybin
GSK Features Results from the P-III (RUBY) Study of Jemperli to Treat Endometrial Cancer at Society of Gynecologic Oncology 2024
Read More: GSK
Asieris Highlights the P-III Study Data of APL-1702 for Treating Cervical High-Grade Squamous Intraepithelial Lesions at the 2024 EUROGIN Congress and SGO Annual Meeting
Read More: Asieris Pharmaceuticals
AstraZeneca Features the P-III (DUO-E) Study Results Evaluating Imfinzi for the Treatment of Endometrial Cancer at SGO 2024
Read More: AstraZeneca
Innovent Highlights the P-II Study Results of IBI302 to Treat Neovascular Age-related Macular Degeneration (nAMD)
Read More: Innovent
Bayer Reveals the P-III (OASIS 3) Trial Results of Elinzanetant to Treat Vasomotor Symptoms in Postmenopausal Women
Read More: Bayer
Jasper Therapeutics Doses First Patient with Briquilimab in the P-Ib/IIa Study to Treat Chronic Inducible Urticaria
Read More: Jasper Therapeutics
Merck Features Data from a Series of P-III Trials Assessing V116 Vaccine for Pneumococcal Diseases at ISPPD-13 2024
Read More: Merck
Merck Reports P-III (KEYLYNK-006) Study Results of Keytruda to Treat Metastatic Non-squamous NSCLC
Read More: Merck
Molecure Doses First Patient with OATD-01 in the P-II (KITE) Trial for Treating Pulmonary Sarcoidosis Across the UK
Read More: Molecure
AstraZeneca Reports Results from the P-III (FLAURA2) Trial of Tagrisso Plus Chemotherapy to Treat Advanced Lung Cancer
Read More: AstraZeneca

The US FDA Grants Approval to Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) for Treating NASH with Liver Fibrosis
Read More: Madrigal Pharmaceuticals
The US FDA’s ODAC Recommends Johnson & Johnson’s Carvykti for the Treatment of Relapsed/Refractory Multiple Myeloma
Read More: Johnson & Johnson
The US FDA Grants Approval to Orchard Therapeutics’ Lenmeldy for Treating Metachromatic Leukodystrophy
Read More: Orchard Therapeutics
Health Canada Accepts for Review Biogen Canada’s New Drug Submission of Tofersen for the Treatment of Amyotrophic Lateral Sclerosis
Read More: Biogen
The EC Grants Approval to BMS’ Abecma for the Treatment of Relapsed/Refractory Multiple Myeloma
Read More: BMS
The US FDA Grants Orphan Drug Designation to Cabaletta Bio’s CABA-201 for Treating Systemic Sclerosis
Read More: Cabaletta Bio
The US FDA Approves Italfarmaco’s Duvyzat (givinostat) for Treating Duchenne Muscular Dystrophy
Read More: Italfarmaco

Fennec Pharmaceuticals and Norgine Join Forces to Commercialize Pedmarqsi in the EU, Australia and New Zealand
Read More: Fennec Pharmaceuticals & Norgine
Sanyou Bio Partners with BioGeometry to Develop AIGC Driven Antibody Drug Discovery Platform
Read More: Sanyou Bio & BioGeometry
Bio-Thera Solutions and SteinCares Join Forces for Marketing Biosimilars Across LATAM
Read More: Bio-Thera Solutions & SteinCares
Evommune Expands its Partnership with Maruho for the Development and Commercialization of EVO756 in Greater China and Asian Countries
Read More: Evommune & Maruho
Bayer and Thermo Fisher Scientific Collaborate on NGS-Based Companion Diagnostic Assays (CDx)
Read More: Bayer & Thermo Fisher Scientific
Kazia Therapeutics Partners with Sovargen to Develop Paxalisib for the Treatment of Intractable Seizures in Rare CNS Diseases
Read More: Kazia Therapeutics & Sovargen
Relief Therapeutics Collaborates with Eton Pharmaceuticals to Commercialize Golike Products for Phenylketonuria
Read More: Relief Therapeutics & Eton Pharmaceuticals

The US FDA Grants Breakthrough Device Designation to Vicore’s Almee for Pulmonary Fibrosis
Read More: Vicore
The US FDA Grants 510(k) Clearance to inHEART’s AI-Based Software Module for Creating 3D Cardiac Models
Read More: inHEART
Onymos and Vapotherm Collaborate for Developing an End-to-End Internet of Medical Things Solution (IoMT)
Read More: Onymos & Vapotherm
TELA Bio Launches its LIQUIFIX FIX8 and LIQUIFIX Precision Devices for Internal Use in Hernia Surgery Across the US
Read More: TELA Bio

For an Aggregate of ~2.4B, AstraZeneca to Acquire Fusion Pharmaceuticals for the Development of Radioconjugates
Read More: AstraZeneca & Fusion Pharmaceuticals
Related Post:- PharmaShots Weekly Snapshots (March 11 – March 15, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














